This is the fifth installment in my series on progress toward the elimination of Hepatitis C infection and disease. Read more about Hepatitis C in part one, part two , part three, and part four.
In previous articles of this series, we’ve discussed several aspects of the hepatitis C virus including the nature of the infection and its distribution both globally and in the United States. Most recently, we’ve discussed the history of direct acting antiviral drugs for the treatment of hepatitis C. Here we describe the use of those drugs to treat the disease. Remarkably, these drugs can not only cure hepatitis C in individuals but also eliminate the disease in entire populations. In this article, we’ll cover the clinical treatment protocols for hepatitis C and its importance in providing optimal care to each patient.
Hepatitis C Diagnostic Tests
Hepatitis C is still endemic to most of the world, but it need not remain so. The disease has been eliminated in several counties, like Egypt, with widespread diagnostic screening and accessible treatment. However, before treatment is initiated, it is critical to properly diagnose and ascertain if treatment is needed at all. Some people with hepatitis C can clear the infection on their own while the majority, if left untreated, can have long term virus replication. These chronic infections can lead to serious liver disease, cancer, and other serious consequences. A streamlined diagnosis process is, therefore, crucial to the United States’ eradication efforts. The first step in hepatitis C diagnosis is to screen for exposure to the hepatitis C virus. Exposure is determined by measuring the presence of hepatitis C antibodies in the blood which are produced whenever a person is infected regardless of whether the infection cleared on its own. This is done by performing a rapid test much analogous to the one used to diagnose COVID-19. These rapid assays use a finger prick and can produce accurate results in as little as 20 minutes. A positive result from this test indicates the presence of hepatitis C antibodies and confirms an infection (Figure 1).

Figure 1. Picture Of A Hepatitis C Antibody Rapid Test. This Test Uses A Fingerpick To Detect Antibodies. The Above Test Is A Non-Reactive Negative Result, And The Bottom Test Is A Reactive Positive Result.
GIDDY
After confirmation that hepatitis C antibodies are present, an RNA test needs to be performed to determine if there is active virus replication. This test uses a polymerase chain reaction to measure the levels of hepatitis C virus RNA in the blood. Again, this test is analogous to the one used to diagnose COVID-19 and is widely available. These tests also determine the levels of viral RNA in the blood also known as the viral load. A high viral load means that the virus is actively replicating, and the infection is current. A small percent of the population, however, may have hepatitis C antibodies present but a low or undetectable viral load. This is mostly likely because the person has undergone spontaneous clearance, or their body’s natural immune system was able to clear the infection on its own. Both the antibody and RNA tests are highly specific and sensitive, making false positives and false negatives rare occurrences. After a confirmed active hepatitis C infection, an additional nucleotide genotyping test should be performed to determine the genotype of the viral infection (Figure 2). Determining both the genotype and viral load will determine the best course of treatment.
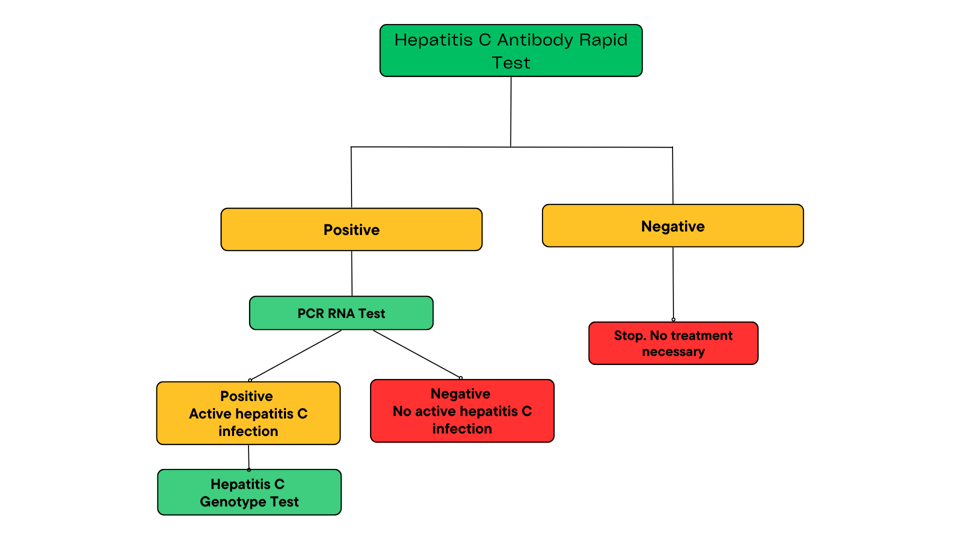
FIGURE 2. THE HEPATITIS C DIAGNOSIS CASCADE. DIAGNOSIS BEGINS WITH A RAPID ASSAY TO DETECT THE PRESENCE OF HEPATITIS C VIRUS ANTIBODIES. A PCR TEST IS THEN PERFORMED TO DETERMINE THE LEVELS OF VIRAL RNA IN THE BLOOD. THE PRESENCE OF BOTH HEPATITIS C
ACCESS INTERNATIONAL
The Right Drug for the Right Genotype
From previous articles, we should be familiar with the importance of matching the right direct acting antiviral to the right hepatitis C strain. For example, with COVID-19, antibodies only work for some but not all virus variants. Hepatitis C, although not as apparently variable in the population, does come in many different variants. Each variant responds somewhat differently to each of the hepatitis C drugs. For example, several of the strains endemic to Egypt and India are relatively resistant to interferon and ribavirin treatments. More recent data has shown that, after a confirmation of active hepatitis C viral replication, it is critical to determine the specific strain of hepatitis C, called the genotype.

FIGURE 3. GLOBAL DISTRIBUTION OF HEPATITIS C GENOTYPES. GENOTYPE 1 IS THE MOST PREVALENT IN MOST REGIONS OF THE WORLD. GENOTYPE 3 IS THE MOST COMMON ON THE INDIA SUBCONTINENT WHILE GENOTYPE 4 IS MOST PREVALENT IN CENTRAL AND NORTHERN AFRICA.
HÉZODE, C 2016 HTTPS://DOI.ORG/10.1111/JVH.12635
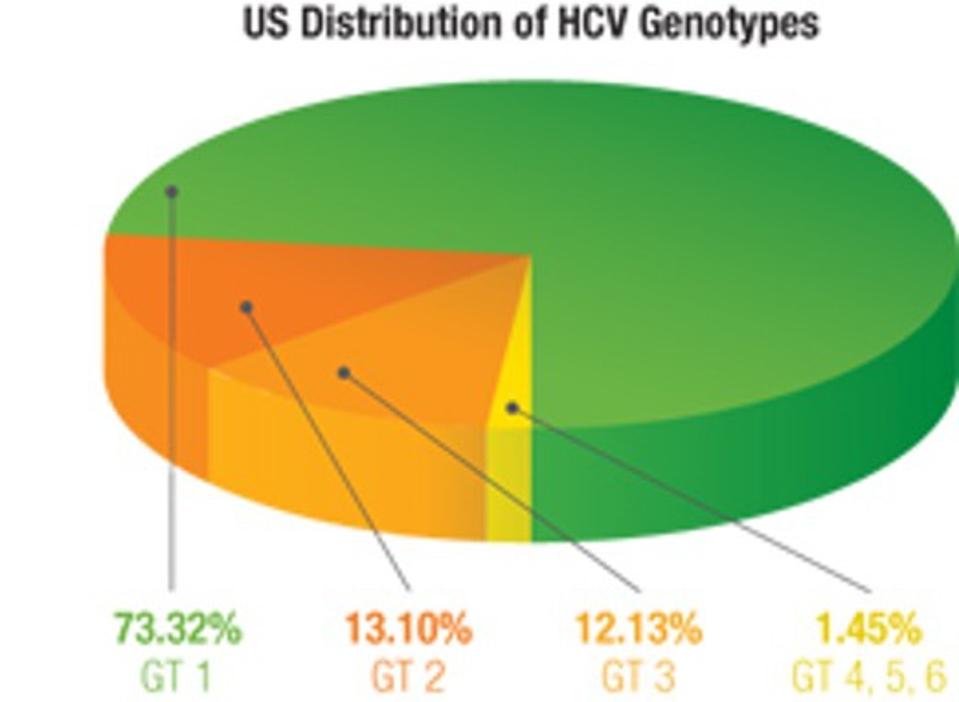
FIGURE 4. DISTRIBUTION OF GENOTYPES IN THE UNITED STATES. GENOTYPE 1 IS THE MOST PREVALENT AND CONTRIBUTES TO ABOUT 73% OF CASES, FOLLOWED BY GENOTYPE 2 (13%), AND GENOTYPE 3 (12%). GENOTYPES 4, 5, AND 6 ARE RARER AND ACCOUNT FOR LESS THAN 2% OF INFE
ABBOTT
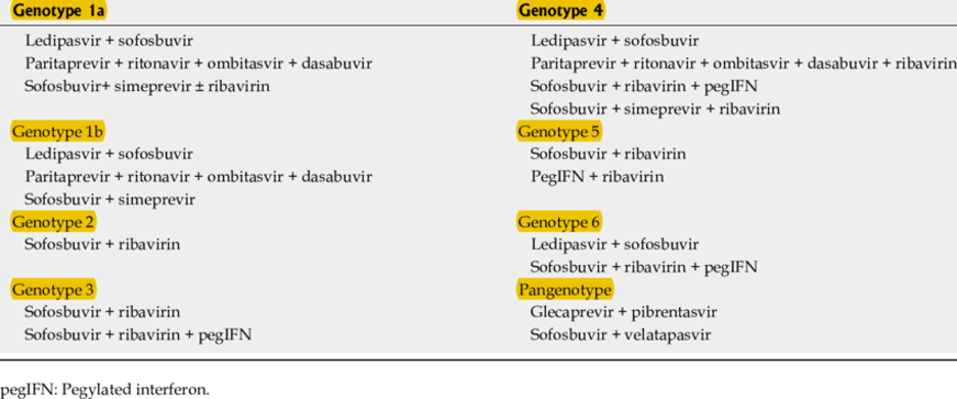
Genotypes are determined using genomic nucleotide sequencing assays. These tests use PCR to amplify and sequence hepatitis C virus RNA to detect sequence variations in the virus’s Core and NS5B protein regions. There are currently 6 different hepatitis C genotypes present across the globe (Figure 3). In the United States, genotypes 1, 2, and 3 make up over 98% of infections (Figure 4). Some genotypes can be further divided into subtypes. Genotype 1, for example, has subtypes 1a and 1b. Genotype can also affect disease progression in addition to treatment response. Those with subtype 1b may be at higher risk for developing cirrhosis, and those with genotypes 1b and 3 have higher chances of developing liver cancer. It is possible to be co-infected with more than one hepatitis C genotype or subtype which is called a mixed infection. Mixed infections may require more robust sequencing methods and more tailored treatment regimens. Recommended treatment regimens by subtype can be seen in Figure 5a, 5b, and 5c. As drug development progresses, many newer direct-acting antivirals are designed to be pangeotypic and can treat all genotypes with equal success.

5A.
THE MEDICAL LETTER ON DRUGS AND THERAPEUTICS
5B.
SALVADORI, M; TSALOUCHOS, A 2018 10.5500/WJT.V8.I4.84
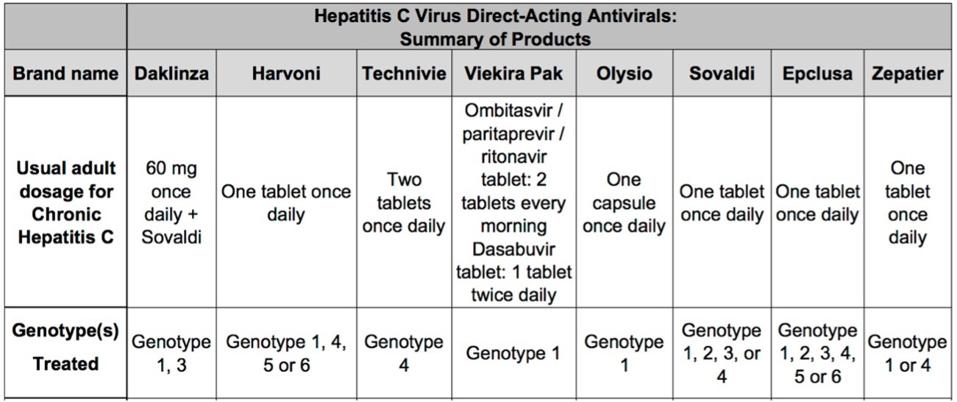
FIGURES 5A, 5B, AND 5C. TABLES OF DIRECT ACTING ANTIVIRAL REGIMENS AND THE GENOTYPES THEY ARE BEST SUITED TO TREAT. MANY REGIMENS ARE GEARED TOWARD TREATING GENOTYPES 1, 2, AND 3 WHICH ARE THE MOST COMMON IN THE UNITED STATES.
PHARMACY CONSULTANTS AND INVESTIGATORS
Treatment Regimens and Outcomes
Most direct-acting antiviral regimens consist of taking a daily pill for an average of 3 months with interval RNA testing performed to assess how well the treatment is working. The end goal of treatment is to achieve a sustained viral response. This is measured by an undetectable hepatitis C virus RNA level, typically under 25 IU/mL, at least 3 months after completing therapeutic treatment (Figure 6). Achieving an undetectable viral load within this timeframe is considered a clinical cure, and 99% of patients who finish a course of direct-acting antiviral therapy achieve a sustained viral response. About 1-2% of patients who are treated with direct-acting antivirals may not achieve a sustained viral response. In those cases, another drug combination may be prescribed and pegylated interferon or ribavirin may be added.
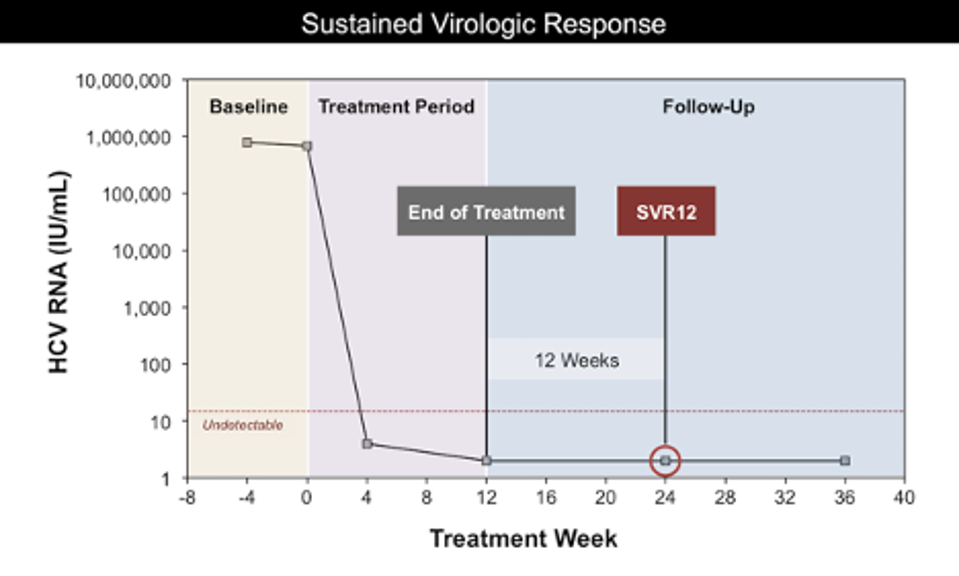
FIGURE 6. A TIME GRAPH OF DIRECT ACTING ANTIVIRAL TREATMENT. VIRAL RNA LEVELS ARE MEASURED AT THE BEGINNING OF TREATMENT AND AFTER A FULL COURSE OF TREATMENT THAT LASTS AROUND 12 WEEKS. IF VIRAL RNA LEVELS ARE STILL UNDETECTABLE 12 WEEKS AFTER THE CO
HEPATITIS C ONLINE
In addition to virus genotype, the screening process for hepatitis C also involves assessing additional risk factors for each patient, especially when retreating a patient who has been previously treated with a direct-acting antiviral-based therapy. In the United States, the current recommendation for starting direct-acting antiviral treatment is to begin treatment within 6 weeks of a confirmed diagnosis. High- risk factors like co-infections with hepatitis B or the human immunodeficiency virus, significant cirrhosis, or the necessity for liver transplantation indicate that immediate treatment is needed. Unfortunately, in some places, treatment with direct-acting antivirals is both expensive and difficult to obtain. These additional calculated risk factors can affect a patient’s likelihood of receiving timely treatment as well as if and which drugs are covered by medical insurance providers.
In conclusion, in the past few decades, we’ve witnessed something close to a medical miracle in the treatment of hepatitis C. The number of drugs and diagnostic tests developed are well studied and highly effective in producing a permanent cure for each individual patient. As we’ll see in the next part of this series, these drugs can also eliminate hepatitis C in whole populations around the world.