This is fourth installment in my series on progress toward the elimination of Hepatitis C infection and disease. Read more about Hepatitis C in part one, part two and part three.
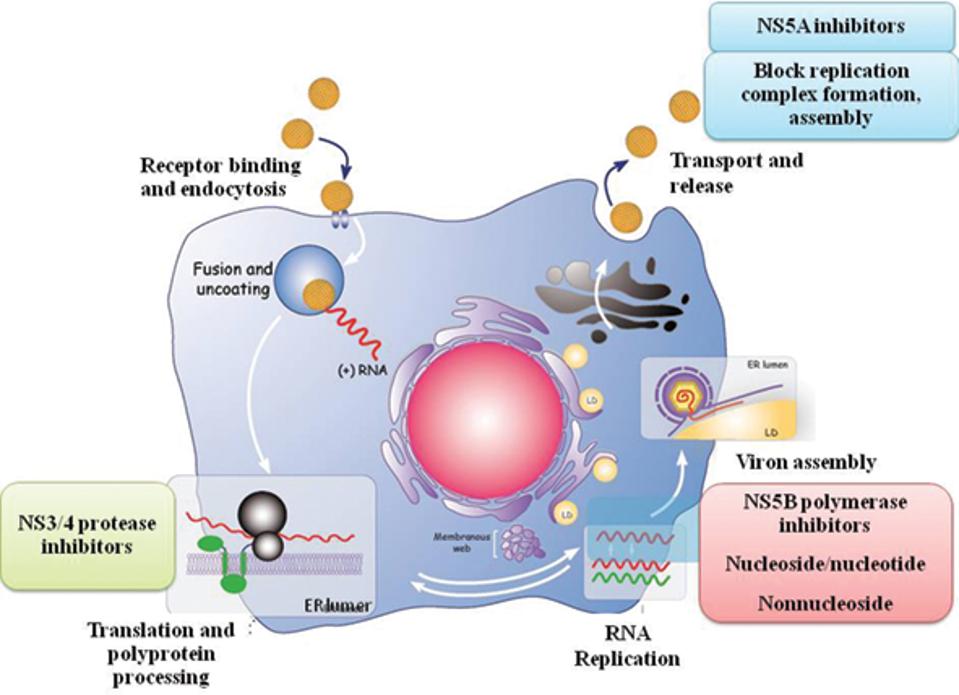
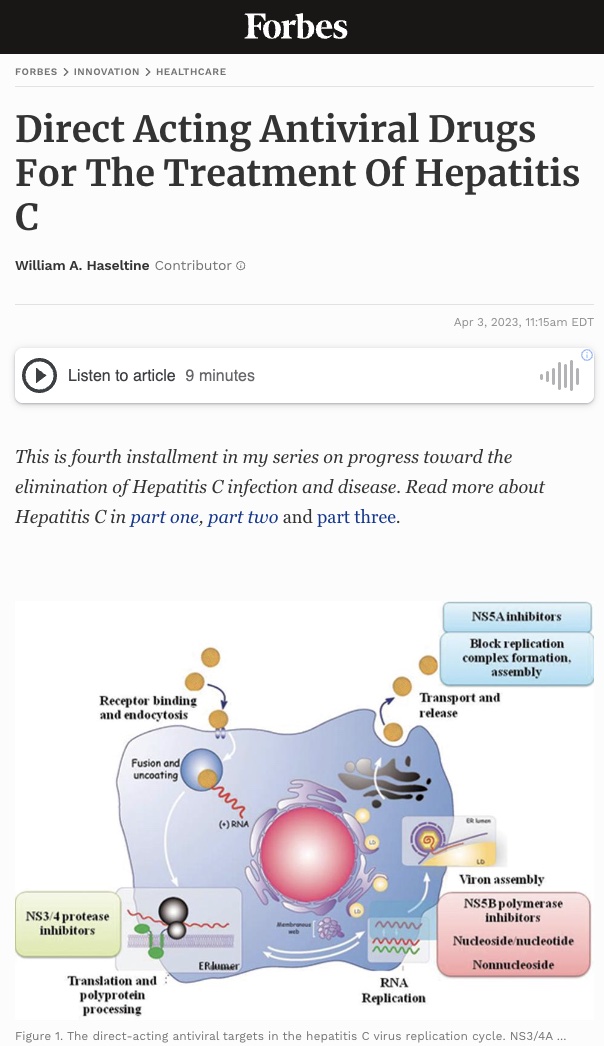
Figure 1. The direct-acting antiviral targets in the hepatitis C virus replication cycle. NS3/4A protease inhibitors target the NS3/4A protease enzyme which is needed for the virus to develop the proteins necessary to replicate itself. NS5B inhibitors work by targeting the NS5B enzyme that initiates the synthesis of the hepatitis C virus’s RNA genomes. NS5A inhibitors work by blocking the virus’s ability to assemble new virions.
KAMAL, S 2017 HTTPS://DOI.ORG/10.1016/B978-0-12-803233-6.00017-5
In the previous articles of this series, I’ve outlined the seriousness and uncontrolled nature of hepatitis C. In the absence of a vaccine, the control and treatment of hepatitis C, much like that of HIV/AIDS, depends on the use of antiviral medications. Recent progress in the development of several highly active, anti-hepatitis C drugs has been a triumph of modern medicine. These drugs usher in an era of effective treatment and even elimination of hepatitis C both nationally and globally. Later in this series I will describe how these drugs have been used to eliminate hepatitis C from some countries and discuss how that success may be replicated elsewhere.
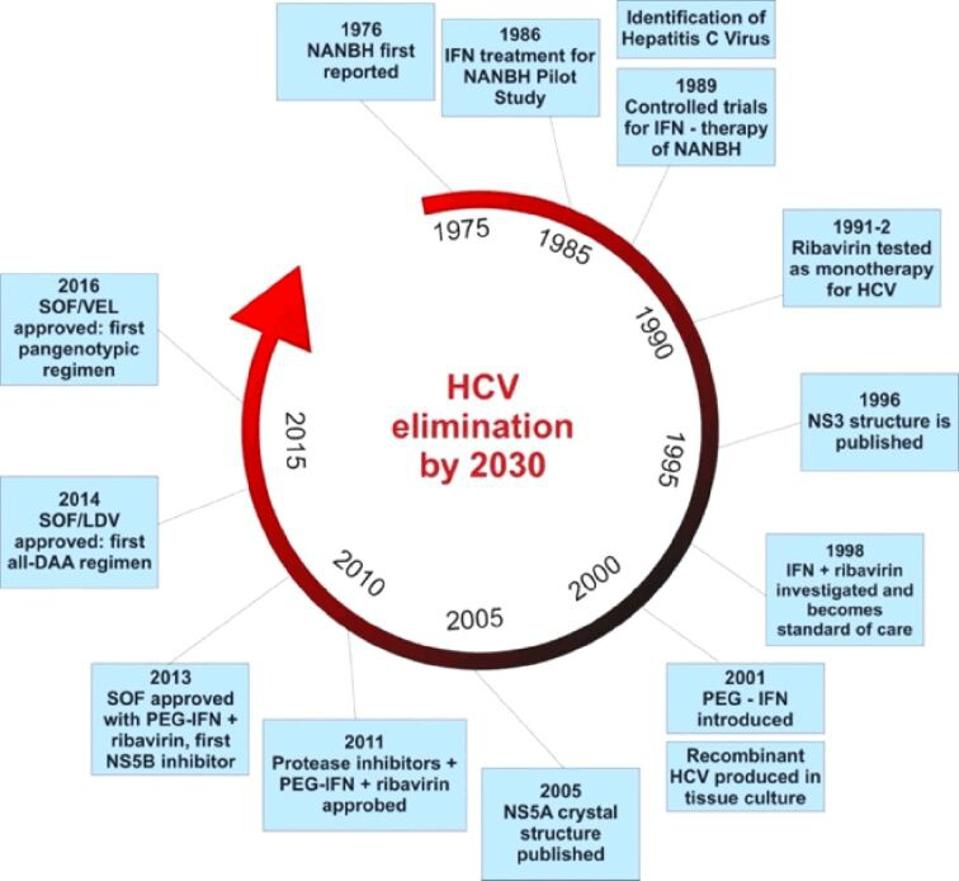
Figure 2. The evolution of hepatitis C drugs. The first available treatment used alpha interferon monotherapy and was followed by combination alpha interferon and ribavirin therapy. The next generation of drugs used long acting pegylated alpha interf
OANCEA ET AL. 2020 DOI:10.47162/RJME.61.3.02
History
The first effective treatment for hepatitis C infection, alpha interferon, was introduced in 1986. Interferons are naturally occurring proteins in the body that mobilize the body’s natural immune system. Interferons do not act directly on the virus itself but rather activate the hosts’ antiviral immune defense. This early version of immunotherapy did have some clinical benefit but was largely ineffective against more advanced hepatic C induced liver disease. Treatment with alpha-interferon is accompanied by serious adverse events including, including hair loss, gum disease, and flu-like symptoms.
In the mid 1990s the drug ribavirin was added to the treatment regimen. Whereas interferon acts by activating the immune system, ribavirin interrupts virus replication, presumably by inhibition of the polymerase. The combination of the two drugs resulted in sustained suppression of the infection for up to half of those treated. The drug combination is not equally effective against all hepatitis C strains. The addition of ribavirin boosted the effectiveness of the alpha interferon therapy but also came with an additional risk of serious side effects including thyroid issues, anemia, and psychosis.
The next generation of hepatitis C drugs appeared in the early 2000s in the form of long-acting pegylated alpha interferons. Pegylation is the process of increasing the molecule size of an interferon by adding polyethylene glycol molecules. The larger interferon molecules slow down the rate in which the drug is absorbed, thus increasing the amount of time the drug remains in the body. These long acting pegylated interferons had higher response rates than earlier interferon therapy, especially when combined with ribavirin. They also needed to be injected fewer times than interferon monotherapy which lessened side effects.
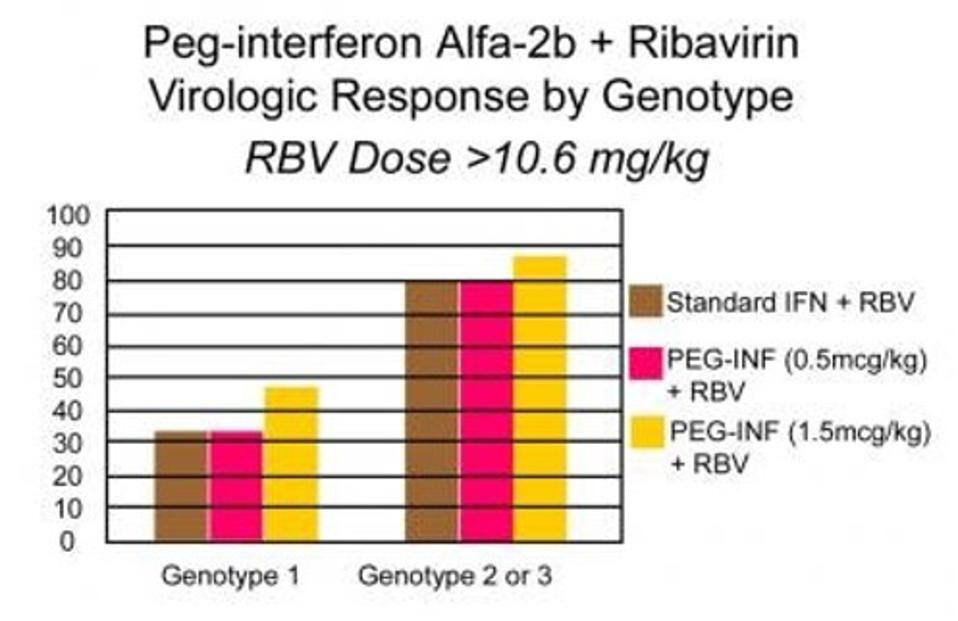
Figure 3. Comparative chart of sustained viral responses to combined standard alpha interferon and ribavirin therapy and combined pegylated interferon and ribavirin therapy by hepatitis C genotype. Up to 80% of patients who had genotypes 3 or 4 and r
DHAWAN, V ET AL 2019 HEPATITIS C TREATMENT & MANAGEMENT
One of the major shortcomings of early hepatitis C drugs was their various effectiveness across different hepatitis C virus subtypes. In the United States, approximately 75% of chronic hepatitis C infections are caused by hepatitis C genotype 1, 15 to 20% by genotype 2 or 3, and less than 5% by genotypes 4, 5, or 6. The combination therapy of alpha interferon and ribavirin increased the efficacy of interferon monotherapy from around 15% to 40%, but response varied by hepatitis C virus genotype. Only an average of 30% of those with subtype 1 were able to achieve a sustained viral response while 70% of those with subtypes 2 and 3 were able to achieve a sustained viral response. The effectiveness of combination long-acting pegylated alpha interferon and ribavirin also varied across genotypes. Treatment was least effective in those with genotypes 1 and 4, and only around half of patients with those subtypes were able to achieve a sustained viral response. Those with genotype 2 and 3, however, had a sustained viral response rate of around 80% and in half the amount of treatment duration.
Current Drugs
As the need for a more curative hepatitis C treatment grew, drug companies began investing in developing a new type of drug—one that could offer a more sustained viral response across all genotypes. This new generation of drugs, called direct-acting antivirals, were designed to attack specific molecular targets known to play a role in the replication of the hepatitis C virus. In 2011, the first direct-acting antivirals for hepatitis C, telaprevir (developed by Vertex pharmaceuticals and Johnson and Johnson) and boceprevir (developed by Merck), were approved by the Federal Food and Drug Administration. These first generation direct-acting antivirals offered a more sustained virological response, especially among those with genotype 1, as well as a shorter duration of treatment, and fewer adverse effects. Today’s direct-acting antivirals are even more effective and are classified by which part of the hepatitis C virus’ replication cycle they target (Figure 1). There are 3 classes of direct-acting antivirals: inhibitors of the NS3/4A protease, those that target the NS5A replicase factor, and those that target the NS5B RNA polymerase.
NS3/4A protease inhibitors target the NS3/4A protease enzyme which is needed for the virus to develop the proteins necessary to replicate itself. These drugs are mainly used to treat genotype 1 of the hepatitis C virus but may also be used in combination with other drugs to attack the virus from multiple replication sites. Compared to other classes of direct-acting antivirals, NS3/4As have longer regimens, more side effects, and are more susceptible to the hepatitis C virus developing a resistance. NS3/4A protease inhibitors currently approved to treat hepatitis C include: Grazoprevir, Paritaprevir, Voxileprevir, and Glecaprevir.
NS5A is a multifunctional protein important for the replication of the viral genome. NS5A inhibitors work by blocking the virus’s ability to assemble new virions. These inhibitors are effective against all virus genotypes but can also be poorly tolerated and are susceptible to resistance. These drugs are also known to work better when combined with either alpha pegylated interferon or ribavirin. Generic names of NS5A protein inhibitors include: Elbasivr, Ledipasvir, Ombitasvir, Velpatasvir, and Pibrentasvir.
NS5B is a membrane anchored enzyme that initiates the synthesis of the hepatitis C virus’s RNA genomes. NS5B inhibitors stop replication of the hepatitis C virus by blocking the function of that enzyme. These drugs work well across all genotypes, are generally well tolerated, and the least susceptible to viral resistance. Current NS5B inhibitors used to treat hepatitis C include: Sofosbuvir and Dasabuvir.
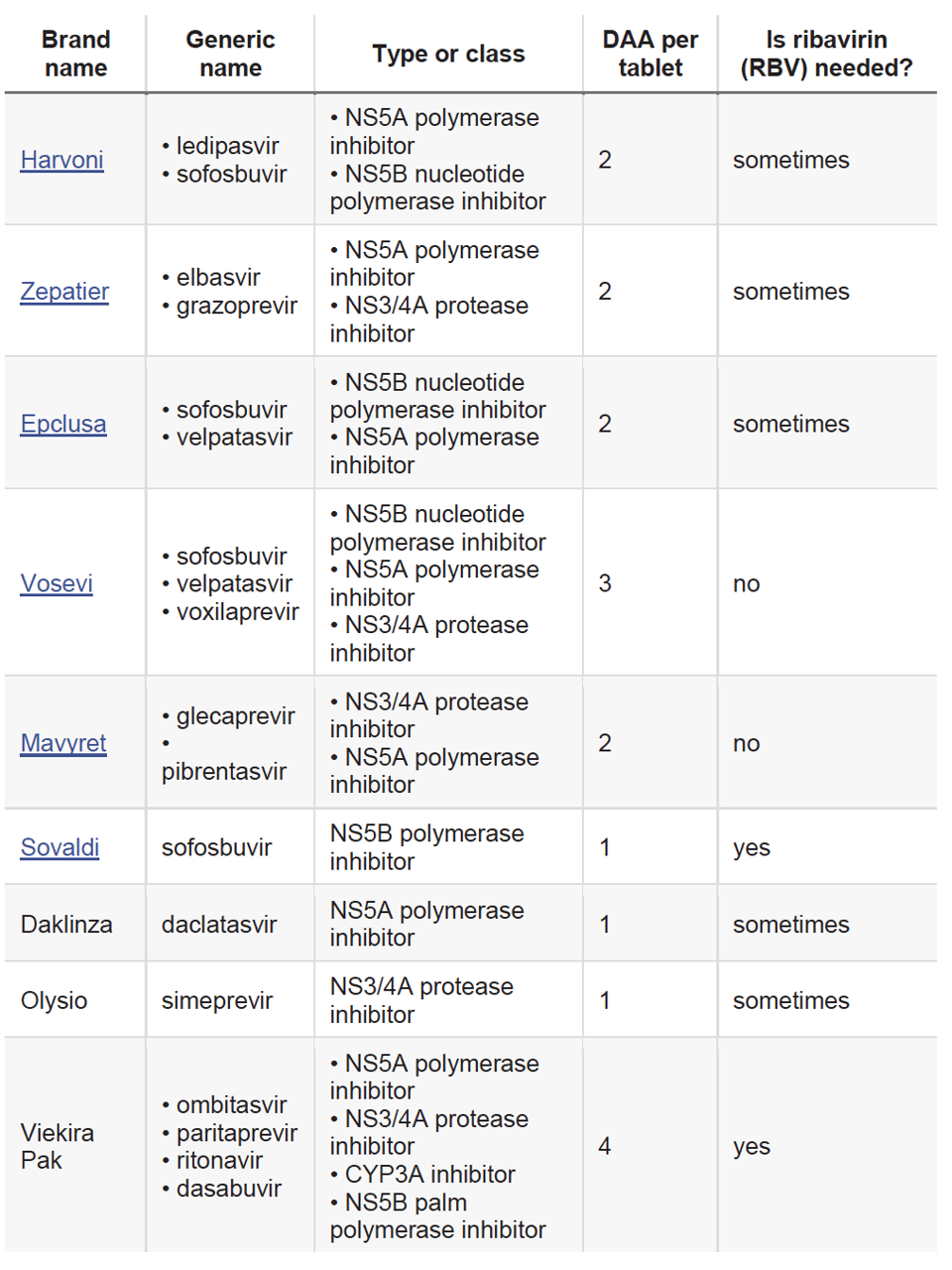
Figure 4. Chart of common direct acting antivirals by brand name and generic name. Many include a combination of direct acting antiviral classes and can be prescribed with or without ribavirin.
MEDICAL NEWS TODAY
Combinations of Direct-Acting Antivirals
Like interferon therapy, optimal treatment with direct-acting antivirals includes consideration of a patient’s genotype and comorbidities such as advanced cirrhosis and coinfection with hepatitis B or HIV. Also, like earlier therapies, many direct acting antivirals are more effective when combined with other antivirals. Much like the human immunodeficiency virus, the hepatitis C virus mutates quickly to develop a resistance to the drugs used to attack it. When used in combination with other antivirals, including ribavirin, direct-acting antivirals can inhibit more than one replication target and increase efficacy. The market for these new combination drugs was also incredibly lucrative. In 2011, Gilead acquired the pharmaceutical company Pharmasset for $11 billion and produced the first hepatitis C combination pill, Harvoni, which was approved in 2014. Harvoni combined two direct-acting antiviral therapies (ledipasvir and sofosbuvir) and was the first FDA-approved regimen that didn’t require the addition of pegylated interferon injections and ribavirin. Today, many other combination pills are available and can be prescribed according to hepatitis C virus genotype.

Figure 5. Recommended hepatitis C drug regimens according to hepatitis C virus genotype. Treatments may include a combination of direct-acting antivirals, ribavirin, and pegylated interferon therapy.
MCLAUGHLIN, M ET AL. US PHARM. 2015;40(4):HS2-HS6.
In the absence of a preventative hepatitis C vaccine, direct-acting antivirals have proven to be very effective in treating active hepatitis C virus infections. Timely, appropriate treatment for hepatitis C, however, remains an important part of disease elimination. In the next part of this series, we’ll cover the clinical protocol for screening and treating hepatitis C with direct acting antivirals.