
This is the ninth article in a 15-part series called “Relevance of Immune Suppression by SARS-CoV-2 to Understanding and Controlling the Covid-19 Pandemic,” which will explore an underappreciated but highly significant aspect of SARS-CoV-2 replication. The ability of SARS-CoV-2 to delay, evade, and suppress the immune system has myriad implications for drugs, vaccines, and other aspects of our pandemic response. The first set of pieces in this series are intended for a general audience; the second set, for the medical community; and the third and final set, for biomedical researchers looking for a deeper understanding of variants, how they’re generated, and what we might do to control them. Read parts 1, 2, 3, 4, 5, 6, 7, and 8.
Closing the door on innate immune signaling
Inhibition of signal peptide membrane transport
Innate immunity not only acts within cells to suppress microbial invasion and signal nearby cells to impending danger, but also initiates the adaptive immune response. To execute this task, the signaling proteins responsible must exit the cell by traversing the cell membrane.
The principal pathway for signaling protein exit is via interaction with a signal peptide sequence located at the amino terminus of the protein. The signal recognition particle contains both proteins and RNA. The signal peptide sequence ferries signaling proteins to a port on the endoplasmic reticulum. It binds to the first peptides, transports them to the membrane, and from there extrudes them into the lumen, eventually allowing the protein to leave the cell when the endoplasmic reticulum fuses with the membrane.
Almost all proteins that exit the cell, including interferons and other signaling molecules induced by interferons, must follow this pathway. Anything that blocks the pathway will prevent signals from the infected cell from exiting to alert nearby cells or activate other components of the immune system.
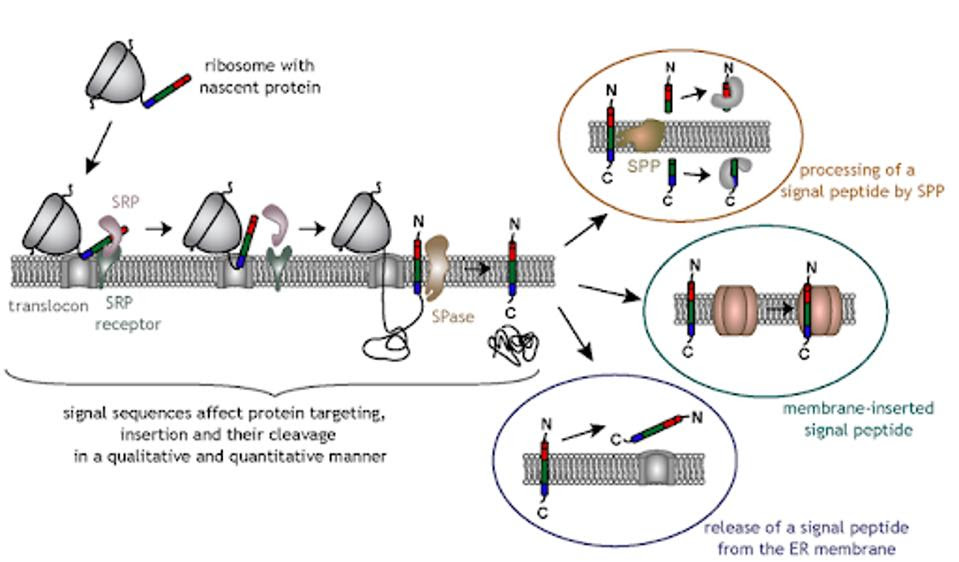
Figure 1: Signal peptide sequence
Evidence suggests that SARS-CoV-2 interference with the transport of signal peptides is mediated by nonstructural proteins NSP8 and NSP9. A study published in October 2020 found that NSP8 and NSP9, both alone and together, bind to the 7SL region of the signal recognition particle, preventing cellular membrane protein trafficking.
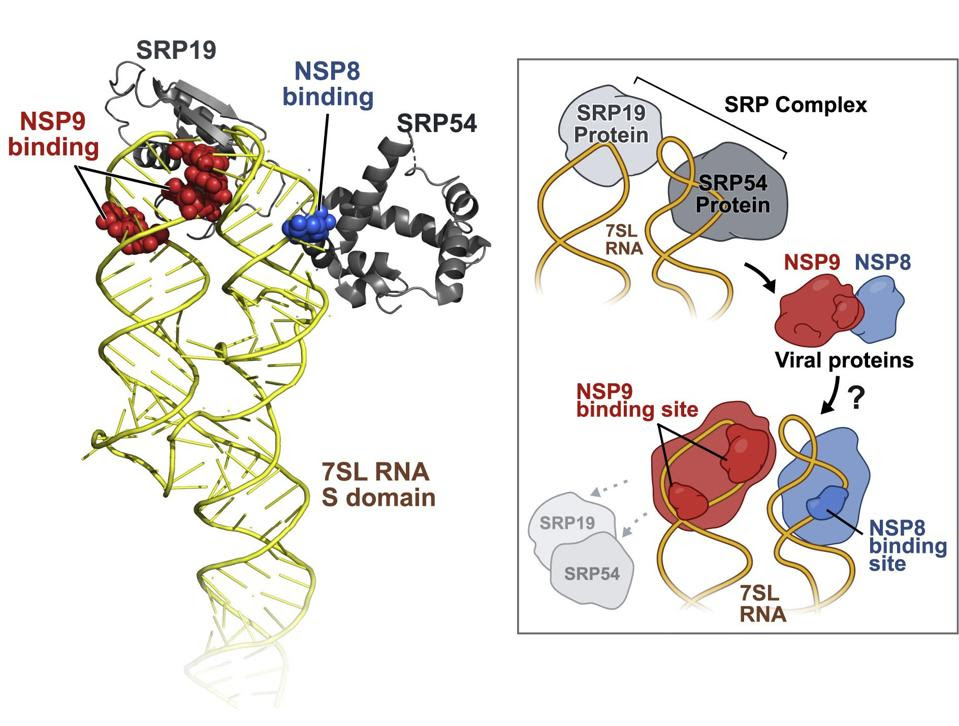
Inhibition of signal peptide export not only prevents an infected cell from signaling its neighbors, but also shields infected cells from T cell recognition and destruction. T cells are the killer cells of the immune system; if they remain unactivated, the virus can replicate to ever higher concentrations.
Downregulation of MHC-I
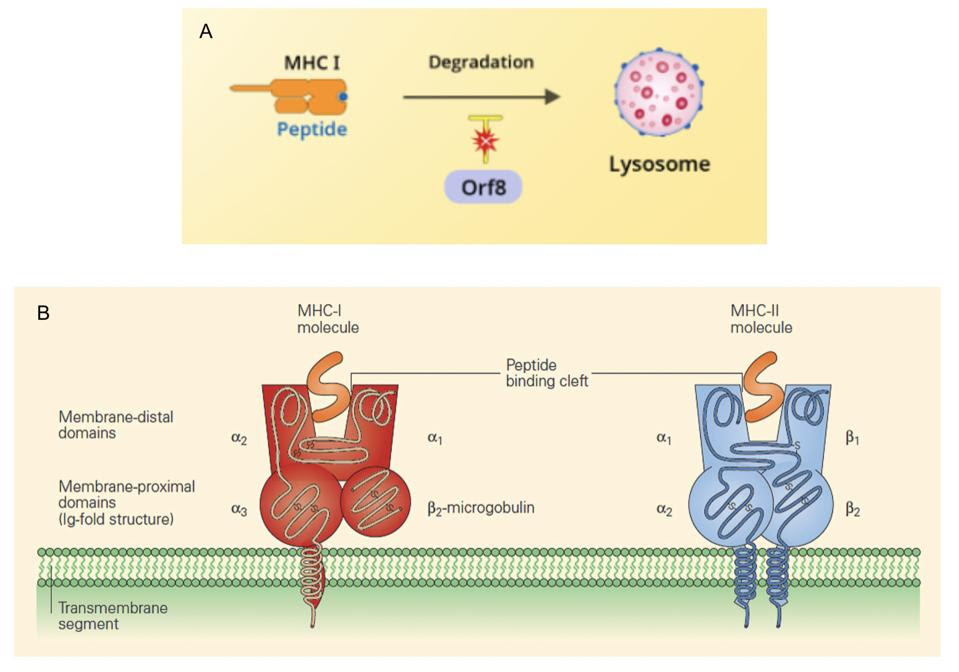
There is another way SARS-CoV-2 disrupts T cell functioning. Molecules classified either as major histocompatibility complex type-I (MHC-I) and major histocompatibility complex type-II (MHC-II) molecules make up cell surface proteins that are involved in stimulating adaptive immunity. MHC-I molecules work by presenting fragments of foreign proteins to T helper cells, teaching them to distinguish between self and not-self and initiating the adaptive immune response. In displaying these peptides, MHC-I molecules become targets for killer T cells.
ACCESS HEALTH INTERNATIONAL AND IMMUNOPAEDIA
In addition to degradation, signal peptide signaling is required for proper processing of the MHC-I proteins. Therefore, SARS-CoV-2 inhibits MHC-I by at least two separate processes.
According to a preprint study from May 2020, the SARS-CoV-2 open reading frame 8 (Orf8) protein obstructs the surface expression of MHC-I. While the exact mechanism is unknown, the authors of the study observed an accumulation of MHC-I proteins in the lysosomes of Orf8-expressing cells, suggesting that Orf8 selectively targets the molecule for degradation and inhibits its involvement in the antiviral response.
SARS-CoV-2 isn’t the only virus to possess MHC-I-targeting mechanisms. The Nef proteins of HIV help the virus also reroute MHC-I molecules for certain destruction, transporting them to the lysosome where they will be degraded, rather than the plasma membrane for export.

