This is part of a series on bystander SARS-CoV-2 induced damage to organs, tissues and cells.
One of the most defining symptoms of Covid-19 is loss of smell without the experience of a stuffy nose, known as anosmia. This distinct feature of Covid-19 affects at least half of those infected with the virus. In most cases, the sense of smell restores itself after a few weeks, but twelve percent of people continue to report complete loss of smell months after the initial infection. A recent study from researchers at the NYU Grossman School of Medicine and Columbia University offers a possible explanation for how structural damage to olfactory cells may delay sensory restoration.
The Indirect Effects of Covid-19 Infection
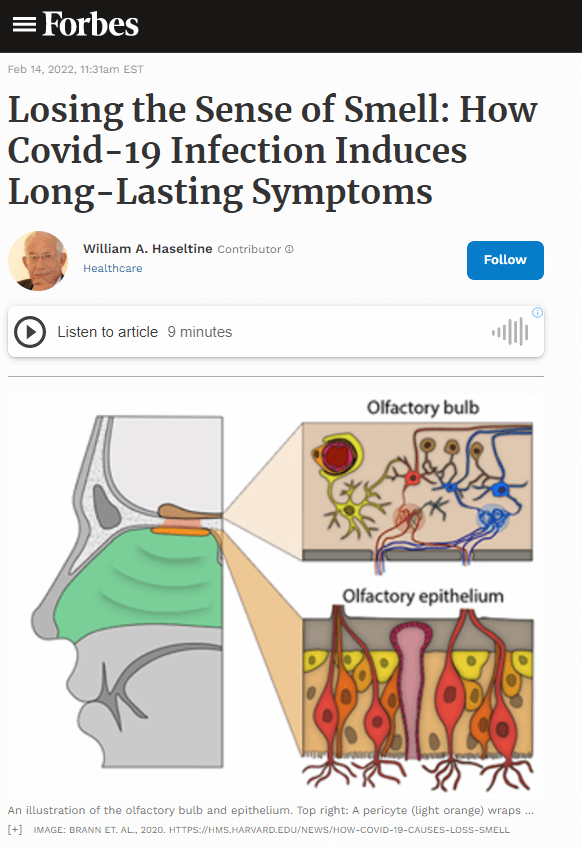
The first insight into how Covid-19 impairs the sense of smell came with the discovery that the virus does not infect olfactory receptor neurons involved in detecting odors. Rather, the virus binds to and depletes sustentacular support cells, which surround olfactory receptors in the epithelium. Unlike receptor neurons, sustentacular cells express ACE-2 and TmPRSS2 membrane proteins that the SARS-CoV-2 uses to attach to and invade cells. Despite the fact that olfactory receptors are not directly infected, they do sustain significant damage from the infection of neighboring cells.
The olfactory bulb is one of the few sensory organs that regenerates its cells. When an olfactory receptor is damaged, the olfactory bulb generates new receptors to replace them in a process called neurogenesis. In contrast to other types of neurons, olfactory receptors regenerate throughout an individual’s life, preserving pathways important for differentiating between various smells. Widespread damage to olfactory neurons, however, can alter or completely erase the sense of smell. In a majority of Covid-19 cases, the sense of smell restores itself with the replacement of damaged olfactory neurons and support cells.
Researchers are just beginning to understand the indirect effects of viral infection on the olfactory system. The next question asks: if the virus does not directly infect olfactory neurons, what explains the damage to nerve cells that contributes to long-lasting loss of smell? Understanding these effects in the olfactory system may provide further insight into how the SARS-CoV-2 virus impacts the nervous system as a whole.
Damage to Olfactory Receptors
To examine the effect, Zazhytska et. al studied infected hamsters, as well as autopsy human samples previously diagnosed with Covid-19. Hamsters serve as ideal models because they rely more on their sense of sense and are more susceptible to nasal infection than humans. Researchers intranasally exposed the SARS-CoV-2 virus to anesthetized hamsters. They studied in detail the effects of the virus in hamsters, then examined post-mortem nasal tissue to see if what they found in hamsters was similar in humans.
Initial observations of the olfactory epithelium detected viral infection in supporting sustentacular cells but not olfactory receptor neurons. Their second analysis took a closer look at the structure of these nerve cells. Much to their surprise they found disruption of a protein network within the neuron’s nucleus, called the olfactosome. The specialized organization of the olfactosome network facilitates interactions between chromosomes important for generating the sense of smell.

When an odor is detected, several membrane proteins on an olfactory receptor are activated called G-coupled proteins. Binding an odor to these receptors induces a sequence of transduction signals that trigger the activity of various proteins and other molecules. These transduction signals tell the olfactory receptors to alert the brain that an odor has been detected. How these cells are able to coordinate these extensive signaling pathways, however, has been a significant area of research. Current theories suggest that the answer lies within the nucleus, where DNA is folded and organized in specific conformations that make up the olfactosome. Olfactory genes are distributed across different chromosomes. To express these genes, chromosomes need to be in close proximity with each other. The olfactosome facilitates these interactions by bringing together distant chromosomes, through a process that is not fully understood.
The destruction of the olfactosome in uninfected neurons explains why the sense of smell disappears. Fortunately, for most people, the sense of smell returns after a couple weeks, though there is no guarantee that it will come back full. The extensive reconstruction of the olfactory epithelium can also prevent receptors from wiring correctly in some cases. This means something that once smelled good may become unpleasant, in a condition called parosmia. After the initial restoration of smell, disordered smell can last anywhere from a few weeks to months.
If the virus does not directly infect these neurons, why does the loss of smell or disordered smell last for so long? Another finding suggests that the key signals that regulate gene expression in the olfactory nerve cell associated with the ability of the neuron to respond to odorants are altered. The process of signal transduction involves several genes important for detecting smells. RNA-sequencing of hamster nasal tissues revealed that Covid-19 infection downregulates, or reduces the activity, of several genes involved in perceiving different odors. Researchers hypothesized that reorganization of the olfactosome disrupts key transcription factors that determine what genes are expressed and when. These changes then alter the orientation and subsequent expression of olfactory genes, which persist even after elimination of the virus forming a sense of “nuclear memory” that delays sensory restoration.
An additional question considered the extent to which viral infection contributes to these abnormalities. In particular, are these changes primarily linked to nearby infection or systemic infection via the bloodstream? By collecting blood serum from infected hamsters and neutralizing the SARS-CoV-2 virus, researchers discovered that immune activity alone induces similar biological responses. This suggests that nearby cells do not need to be infected to see an impact on olfactory neurons. Rather, components of the immune response that circulate the bloodstream, such as cytokines, chemokines, and other immune products, can cause widespread damage.
Lastly, researchers asked whether these effects are similar in humans by examining cadaver nasal tissue. Their investigations confirmed the downregulation of olfactory receptor genes and components of signal transductions that contribute to loss of smell. The downregulation of olfactory receptors and signaling genes provides a likely explanation for prolonged loss of smell following Covid-19 infection. However, we cannot exclude possible biological factors specific to hamsters that may interfere with this theory.
Future Directions
The conclusions from this study demonstrate that “the sense of smell relies on extremely ‘fragile’ genomic interactions between chromosomes,” as stated by co-corresponding author Benjamin tenOever, PhD, professor in the Departments of Medicine and Microbiology at NYU Langone Health. Although this provides an explanation for the persistence of lost sense of smell, further investigations are needed to understand why recovery of olfactory neurons distorts smell and more importantly, how long-haul Covid-19 symptoms impacts brain tissues.
Studies suggest that some cytokine proteins, which coordinate inflammation in response to a pathogen, can cross the blood-brain barrier and enter the cerebrospinal fluid. Therefore, there are significant concerns that viral infection may interfere with cognitive functions. This could explain why some individuals exposed to the virus experience brain fog, headaches, and other neurological effects anywhere from a couple weeks to months after infection. Similar to the pattern of infection seen in the olfactory system, the neurons themselves are not infected but rather the epithelial support cells neighboring brain cells. Since these effects cannot be ethically studied in humans, exploring these cognitive effects in hamsters and other model organisms provides the best insight into how the virus can impact our brains.