Viral variation has proved to be a critical weak point in our approach to medical solutions for controlling Covid-19. Over the last two and a half years, we’ve seen successive waves of reinfection by new variants of those who’ve been previously infected, those who have been vaccinated and boosted, and those who have been infected, vaccinated, and boosted as well. Behind this unfortunate dynamic is the dramatic variation in the structure of the virus exterior, specifically the Spike protein, which plays a critical role early in infection by binding to the cell surface and forcing entry.
Antibodies that recognize this structure can block infection. However, changes in the exterior structure negate antibody collections in convalescent sera and monoclonal antibodies from binding and neutralizing the virus. A recent study by Yamasoba et al. summarizes the effectiveness of existing monoclonal antibodies against a successive set of virus variants, namely the BA.2 variant, which first emerged in late 2021 and quickly spread around the world, driving the most infectious wave of the virus to date, BA 4/5, which are the predominant strains circulating at the time of writing, and BA.2.75, a new sublineage of BA.2 which is likely more infectious and immune evasive than its predecessors, suggesting it may be the predominant variant in the coming weeks and months.
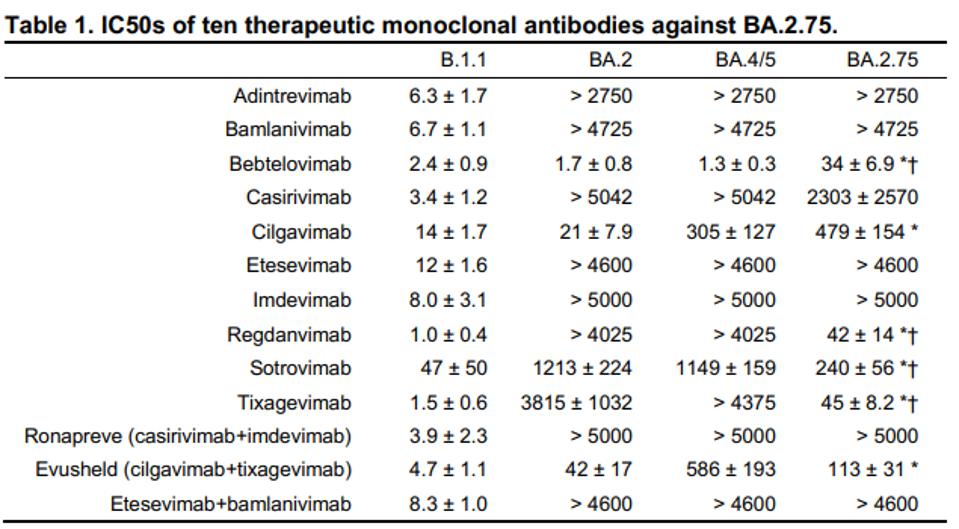
The ability of the virus to evade natural immunity from the previously infected and vaccinated is also reflected in its ability to escape a host of specific monoclonal antibodies. As is clear from Figure one, the later Omicron viruses evade monoclonal antibodies much more effectively than early strains.
Immediately, we note that five antibodies: adintrevimab, bamlanivimab, casirivimab, etesevimab, and imdevimab failed to neutralize any of the three Omicron sublineages. Casirivimab and imdevimab, as well as etesevimab and bamlanivimab, are designed to be used in tandem in an antibody cocktail, yet their combination antibodies were just as ineffective. Adintrevimab is intended for individual use, meaning its neutralization potency for the strains circulating today is nonexistent. These were among the first monoclonal antibodies developed, rationalizing why they are so ineffective against recent strains.
This leaves five individual monoclonal antibodies. Regdanvimab, sotrovimab, and tixagevimab did not neutralize the previously circulating BA.2 and the currently circulating BA.4/5. However, the three effectively neutralized the BA.2.75 pseudovirus. This suggests that if BA.2.75 became the dominant strain in the coming weeks and months, these three monoclonal antibodies could be effective treatments for those suffering from Covid due to this strain.
Of the two remaining antibodies, cilgavimab poorly neutralized BA.2 and BA.4/5 but was 24.4-fold worse against BA.2.75. Although bebtelovimab effectively neutralized BA.2 and BA.4/5, it again was much worse against BA.2.75, this time 21.2 to 25.6-fold. Despite poorly neutralizing BA.2.75 compared to BA.4/5, bebtelovimab still neutralized the strain better than any other antibody.
Even newer generations of viruses recently detected in South Africa with more extensively mutated Spike proteins, against which bebtelovimab and others may perform even more poorly.
New variants evading monoclonal antibodies should come as no surprise. After infection, the convalescent sera of a recovered patient contains many antibodies designed to inhibit the virus the host just overcame. For the virus to reinfect, it must mutate considerably to evade the convalescent antibodies. Monoclonal antibodies are effectively the same as convalescent antibodies on an individual scale. They are designed to overcome a virus by binding to specific amino acids on the Spike. If the virus mutates enough, the monoclonal antibody can no longer bind. This is how the cat and mouse game of developing antibodies and the virus mutating has continued for two and a half years.
What then can be done? The search is on for monoclonal antibodies that recognize regions of the virus that are critical to the virus lifecycle and therefore are resistant to most mutations. In other words, scientists worldwide are rushing to identify and develop antibodies with broadly neutralizing capabilities, i.e., antibodies that recognize highly conserved sequences of the Spike protein that may overcome all viral variants.
The good news is that many such antibodies have already been identified. We recently described the Cv2.1169 antibody discovered by scientists at the Pasteur Institute and will continue to detail others as data is released. Whether these antibodies recognize and neutralize the latest variants such as BA.2.75 remains an open question.
A second potential solution is to use extensive combinations of functional monoclonal antibodies. While many fail to neutralize, some retain neutralizing capability against the latest variants, and new monoclonal antibodies are constantly advancing. Combining two, three, or four antibodies into a single treatment may suppress infection. Our hope remains high for monoclonal antibodies as a short-term relief for those infected and, in the long run, as a prophylactic against infection in the first place.