This story is part of a series on the current progression in Regenerative Medicine. In 1999, I defined regenerative medicine as the collection of interventions that restore tissues and organs damaged by disease, injured by trauma, or worn by time to normal function. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
In this subseries, we focus specifically on gene therapies. We explore the current treatments and examine the advances poised to transform healthcare. Each article in this collection delves into a different aspect of gene therapy’s role within the larger narrative of Regenerative Medicine.
In modern medicine, “Gene therapy has opened a new door, especially for conditions caused by genetic factors. Metachromatic leukodystrophy, MLD for short, is a rare but highly debilitating neurodegenerative disease. It is noteworthy for its difficulties and the potential promise the latest research offers regarding its treatment and possible cure.
A recent publication in Molecular Therapy presents a groundbreaking dose-response evaluation of intravenous gene therapy in a symptomatic mouse model of MLD. This study is a significant leap forward, showcasing the immense potential of genetic intervention strategies to combat and potentially reverse the debilitating effects of MLD.
What is Metachromatic Leukodystrophy?
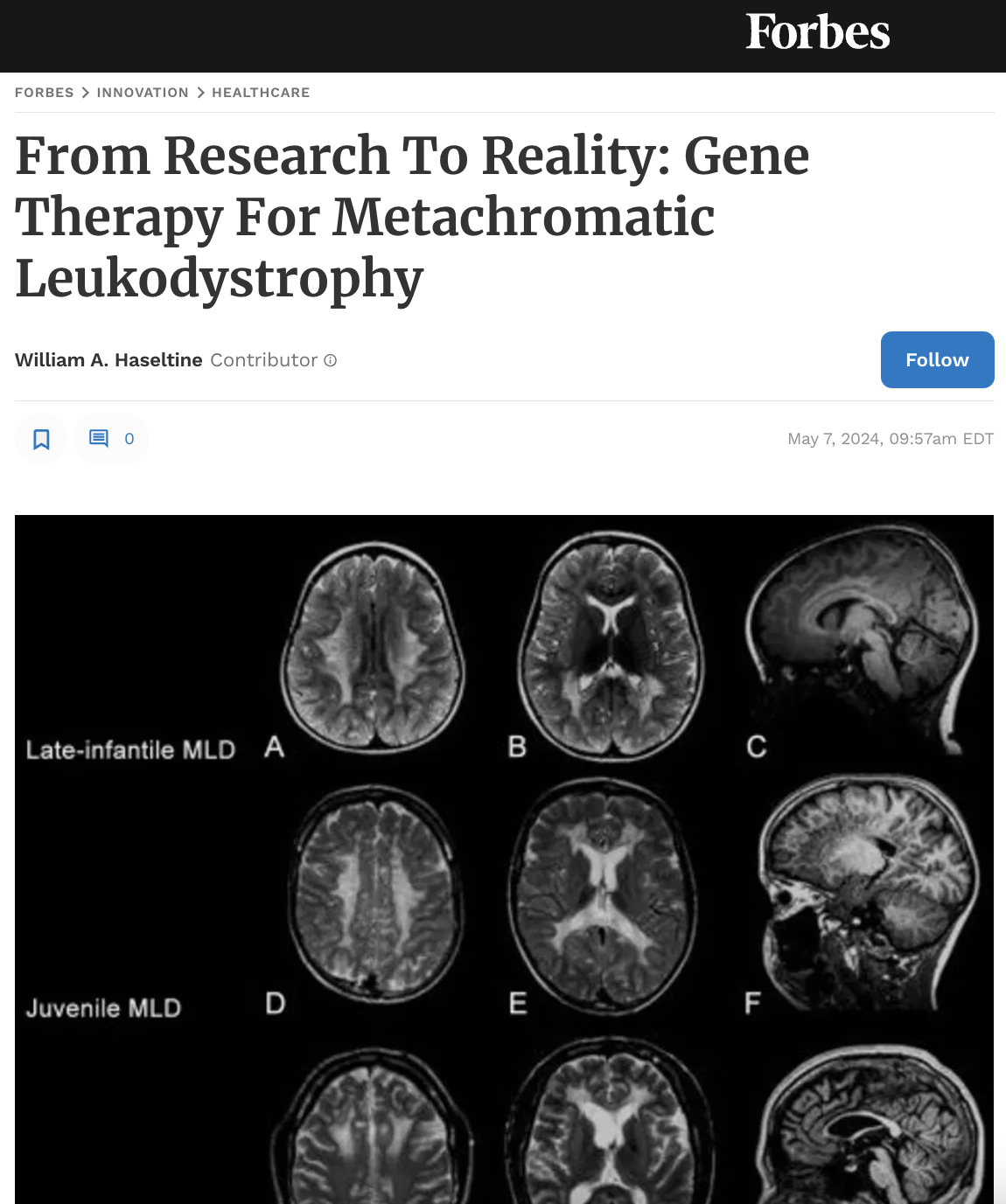
Metachromatic leukodystrophy is a rare genetic disorder that affects the nervous system. This condition is caused by a deficiency of the lysosomal enzyme arylsulfatase A (ARSA), which breaks down sulfatides in the body. When ARSA levels are low, sulfatides build up in the body and cause progressive demyelination of the central and peripheral nervous systems.
MLD causes various symptoms, such as muscle stiffness, weakness, seizures, impaired cognitive function, and loss of vision. The severity of symptoms and age of onset categorize MLD into three types: late infantile, juvenile, and adult. Unfortunately, there is currently no cure for the disease. Still, treatments like enzyme replacement therapy and hematopoietic stem cell transplantation can help alleviate symptoms and slow down the progression of the disease. More recently, studies have focused on the treatment options for gene therapy.
A Gene Therapy On the Horizon
The study has introduced a new adeno-associated virus named PHP. eB (AAVPHP.eB) vector that carries the ARSA enzyme. This vector has shown promising results in treating the disease in a mouse model. The gene therapy approach has corrected the sulfatide storage anomalies. It has the potential to transduce broadly across central nervous and peripheral organs.
Caption: graphical representation of the study
In the study, the researchers employed quantitative PCR analyses to meticulously measure the amount of vector genome copies in various tissues. Their approach enabled them to determine the vector’s successful gene transfer abilities, particularly in critical areas such as the brain and spinal cord, which are often difficult to target with traditional therapies. The findings showed that the vector can efficiently transfer genes to these areas, a promising sign for future therapeutic applications.
The Significance of the Study
The study is a significant step toward translating gene therapy from theoretical models and preclinical studies into a clinically viable treatment avenue for MLD. By demonstrating the effective correction of sulfatide storage in the brain and spinal cord through a non-invasive administration route, the research opens up new doors for tackling not just MLD but potentially other neurodegenerative and lysosomal storage diseases.
Furthermore, quantitative PCR’s application in this context is valuable for assessing gene transfer efficiency, offering insights that could refine and enhance future gene therapy endeavors. This approach augments our understanding of gene therapy’s dynamics. It paves the way for more precise and tailored therapeutic interventions.
Looking beyond the immediate results, the study’s broader implications stir anticipation and hope. It propels the gene therapy field forward, presenting a template that could revolutionize the treatment of neurodegenerative and genetic diseases. By embodying a convergence of strategic innovation, scientific rigor, and practical applicability, this research exemplifies how the challenges posed by rare diseases like MLD can spark pioneering solutions that extend well beyond their initial scope.
A Step Toward Direct Injection Treatments
One of the principal drawbacks or barriers to the wide use of cell modification therapies for cancer, autoimmune disease, and gene therapy is the requirement for the use of autologous cells. Many treatments outlined today require isolating the target cell from an individual, cultivating it in culture, modifying it through gene manipulation, pre-treating the patient, and reintroducing the altered cell back into the patient. Immune ablation is usually required to enable the implantation of modified cells. However, this procedure is intricate, time-consuming, costly, and sometimes risky. In certain instances, immune ablation poses a danger to the patient.
These drawbacks create a severe bottleneck in adopting such cell-mediated and gene therapies. This report offers at least a glimmer of hope that direct injection of specific cell modification check methods may overcome these limitations and might overcome the limitations of the requirement for autologous cell reimplantation. A modifying vector was injected directly into the animal for beneficial effects. There was no necessity for the injection to be autologous. The vector appears to target the appropriate cells effectively in the animal.
In an upcoming essay for Inside Precision Medicine, I highlight the significance of direct injection as a primary focus in cell modification therapies. This method has garnered considerable attention in various research labs worldwide, indicating its potential for self-directed injection.
A New Foundation in MLD Treatment
This dose-response evaluation’s exploratory mission signals a shift in our approach to managing MLD and similar genetic disorders. While the path to clinical application remains fraught with challenges—ranging from regulatory hurdles to the need for broader efficacy and safety studies—the foundation laid by this research inspires a cautiously optimistic outlook.
The melding of gene therapy’s potential with pragmatic, patient-friendly administration methods heralds a new era in treating complex genetic conditions. As we stand on the brink of such medical advances, the collaborative spirit of researchers, clinicians, and patients will undoubtedly be the linchpin in turning these scientific insights into tangible health benefits. Our collective pursuit of innovation, underpinned by studies such as this, lights the way toward a future where genetic diseases no longer dictate terms of life but instead become manageable aspects of it.
To learn more about regenerative medicine, read more stories at www.williamhaseltine.com