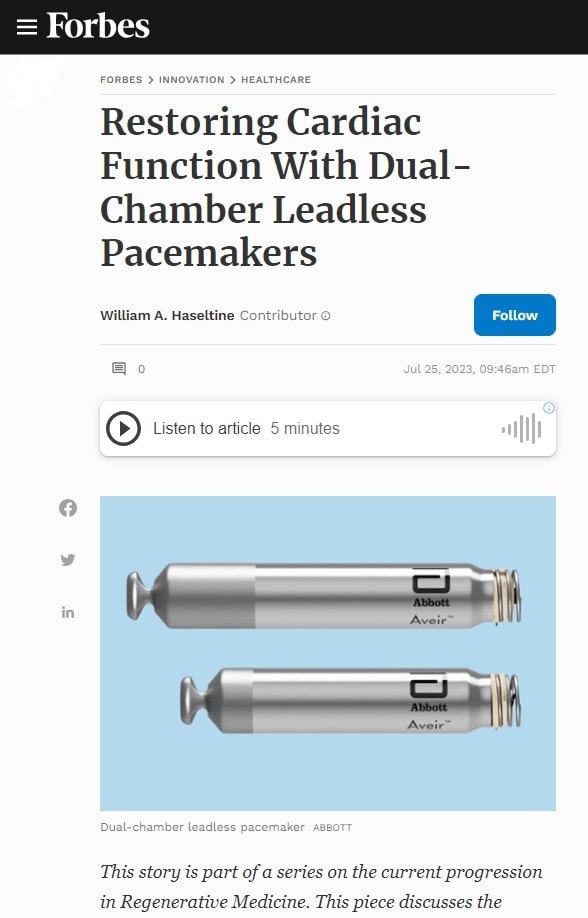
This story is part of a series on the current progression in Regenerative Medicine. This piece discusses the regeneration of the cardiovascular system.
In 1999, I defined regenerative medicine as the collection of interventions that restore to normal function tissues and organs that have been damaged by disease, injured by trauma, or worn by time. I include a full spectrum of chemical, gene, and protein-based medicines, cell-based therapies, and biomechanical interventions that achieve that goal.
A new dual-chamber model pacemaker may increase access to leadless pacemakers by 400%. Yale Medicine estimates that up to three million Americans live with pacemakers. Traditional pacemakers use a lead and pocket system, often associated with transvenous pacing health complications. In the past several years, the single-chamber leadless pacemaker was developed to reduce lead and pocket complications.
The leadless pacemaker reduces the risk of infection by eliminating the use of subcutaneous pockets. The leadless model is more aesthetically appealing by not requiring chest incision, while also being compatible with MRI machine imaging.
While the single-chamber model reconciled the lead and pocket complications, this style did not provide atrial pacing or consistent atrioventricular synchrony, limiting the therapy to roughly 20% of those who require pacemaker intervention.
In recent months, Abbott Laboratories unveiled a dual-chamber model of the leadless pacemaker, overcoming the atrial limitations of the single-chamber model. Dr. Reinoud Knops from the Amsterdam University Medical Centers and colleagues analyzed the safety and efficacy of the Aveir pacemaker in a trial of 300 patients. Here I describe their results and discuss the implications of the novel intervention.
Aveir is a modular two-part system. One half is implanted in the heart’s right atrium and the other in the right ventricle. A dedicated retrieval catheter is also installed to retrieve the pacemakers for diagnostics or repairs efficiently. The two halves of the system communicate wirelessly to synchronize beats and ensure stable heart rates for the patient.

FIGURE 1: Dual-Chamber Leadless Pacemaker System.
KNOPS ET AL.
Over six months in 2022, 300 enrolled patients underwent implantation surgery across 55 centers in the United States, Canada, and Europe, with the average patient being roughly 70 years old. The surgical implantation was successful in 295 patients, with three remaining patients unable to proceed due to inadequate implant-to-implant communication (1%).
Aside from these three patients, 32 complications occurred in 29 patients within three months following surgery. A vast majority (80%) occurred within days of the operation, typically cardiac arrhythmia or dislodgement of the devices. There were four deaths recorded during the study. However, none were considered to be procedurally related.
A total of 271 patients (90.3%) were free from complications three months following surgery, exceeding their goal of 78%. Adequate atrial pacing was recorded in over 90% of patients, and over 97% had sufficient atrioventricular synchrony. These endpoints are critical as the more commonly available single-chamber leadless pacemaker fails where the double-chamber model succeeds.
This study is fascinating for those needing a pacemaker intervention but previously unqualified for the single-chamber leadless model. Dr. Knops and colleagues estimate that only 20% of those with pacemaker requirements could receive the single-chamber model, meaning the dual-chamber will expand availability four-fold.
In addition to being more effective and more widely available, the intervention is safe for the vast majority of patients. In 90% of cases, post-surgical complications are nonexistent, and most needing follow-up surgery do not experience adverse health effects.
One item of note is the eventual cost of the intervention. In the United States, the pacemaker itself and accompanying medical procedures out-of-pocket could cost tens of thousands of dollars. While the dual-chamber model represents a step forward in efficacy and safety, it may also be more expensive with the two-device system. Though such a device could be life-saving, it should not put the patient into a lifetime of medical debt.
While the Aveir pacemaker has received approval from the Food and Drug Administration, the system has yet to be approved abroad. I would expect this to change in the near future, given the positive results from early studies presented by Abbott, and the company’s strong foothold worldwide in medical technologies.
An alternative option for those interested in the Aveir system is the biventricular pacemaker, which uses not two, but three leads in the right ventricle, left ventricle, and right atrium. This model is best for those needing cardiac resynchronization therapy, common in those with heart failure and ventricular desynchrony.
Ultimately, however, the device is a promising step forward for cardiac regenerative medicine. I look forward to seeing how the system progresses in the common months and years, culminating in its sooner rather than later public availability.
To read more of this series, please visit www.williamhaseltine.com