Glioblastoma is one of the most deadly and recalcitrant cancers to impact the brain. The cancer forms masses of tumors on the surface of the brain while concurrently migrating and rooting itself in the neural pathways. Patients with glioblastoma—children and adults alike—are plagued with headaches, nausea, seizures and more. Worse still, the few immune therapies that exist are ineffective, harshly impact the brain, and create spillover effects on cognition, mood, behavior and bodily functions.
The average survival rate has hovered, unchanged, at around eight months for many years. A study published in the journal Science Translational Medicine begins to build on an alternative which may invigorate the field.
Researchers from the Brigham and Women’s Hospital tested a Trojan horse approach: using cancer cells to fight cancer itself. The team investigated the efficacy of their dual-function cancer vaccine on mice with glioblastoma and found potential in this unique method. With the first steps paved, the hope is to continue this path of research and reach clinical translation for humans.
Current Limitations of Cancer Cell-Based Therapy
Killing cancer with cancer sounds deceptively simple, but in fact is a concept under thorough investigation. Typically, cancer cells dodge and suppress the immune system to grow largely unhindered. But cancer cells such as glioblastoma cells have the unique predilection to attract cells of a kind. Using this feature against itself, the Trojan horse method aims to borrow that honing ability to accurately find and destroy cancer itself.
Researchers also see promise in using neoantigens—tumor-specific biological tags found on the surface of cancer cells—in cell therapies to boost immune responses which normally are quelled by cancer. The cell therapy ideally introduces these biological tags to immune cells; with the target recognized and memorized, the immune system stirs to action and begins a fight against tumor cells that share the same neoantigens.
This dream is not yet realized. Both inactivated and live tumor cells have limited success in treating and preventing cancer. Tumor cells inactivated by lysis or irradiation can encourage immune cells to travel to tumor sites, but they lack killing power; the resulting antitumor response thus proves too weak or indirect to be clinically viable. In contrast, living tumor cells can home and target tumors, but often die prematurely by their own toxicity. The answer to cancer cell-based therapy may lie in the gap between living and inactivated tumor cell performance.
Investigating Bifunctional Cancer Cell Vaccine
The team at Brigham and Women’s Hospital noticed the need for cancer cell-based therapy to join the homing and cytotoxic abilities of living tumor cells with the robust immune responses evoked from inactivated tumor cells. Correspondingly, they created a bifunctional cancer cell-based therapy using gene editing.
The researchers designed the cells to release interferon-β (IFN-β), an immune chemical that directly inhibits tumor cell proliferation and the formation of new blood vessels in tumors. To do this, they took living tumor cells from mice and used CRISPR-Cas9 gene editing to knock out interferon-β-specific receptors. By knocking out the receptor gene, the team programmed the cells to produce interferon-β (IFN-β) without fearing autotoxicity.
To bolster indirect immune responses, the team engineered the tumor cells to also express another immune chemical called granulocyte-macrophage colony-stimulating factor (GM-CSF). This growth factor promotes the maturation and proliferation of dendritic immune cells (see Figure 1) which, in turn, prime the immune system for long term antitumor responses.
Lastly, the team incorporated a safety switch. Using cancer as a treatment brings potential risks if uncontrolled, and could possibly initiate unwanted secondary tumors. The switch, made of two enzymes, triggers a double suicide system that ultimately leads to tumor cell death before the cell can proliferate. A graphic summary of the study procedure can be seen in Figure 2.
FIGURE 1: Illustration of a dendritic cell before and after maturation. These cells play a crucial role in immunity due to their ability to cross-present antigens to T cells. They also express costimulatory molecules and produce inflammatory chemicals.
DESIGNUA / SHUTTERSTOCK
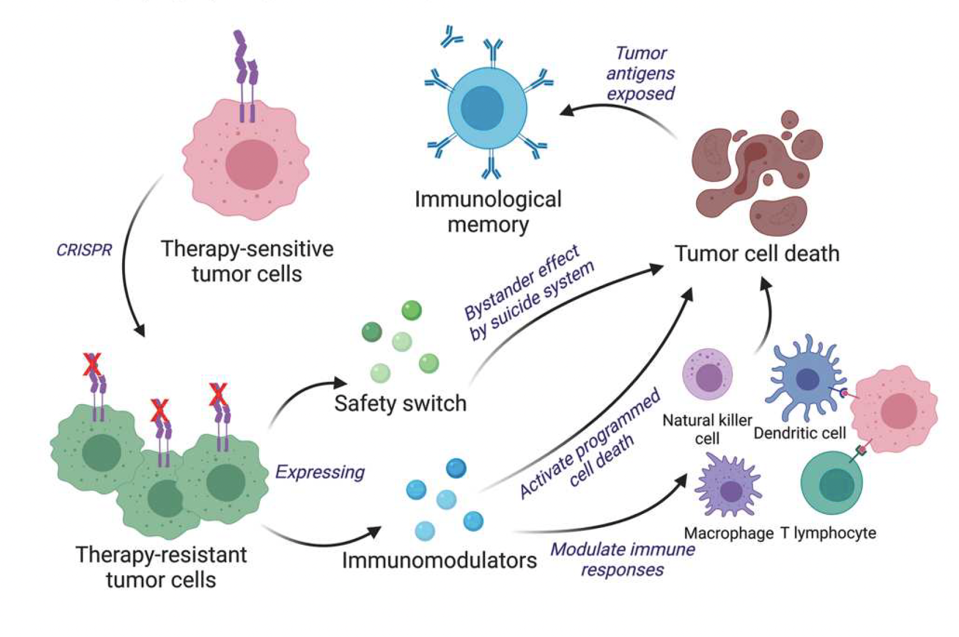
FIGURE 2: Overview of study procedure. In this study, researchers used CRISPR gene editing to alter tumor cells to counter cancer. The genetic changes allow the cells to express chemicals that stimulate immune responses, alongside a safety switch that prevents secondary tumor initiation.
CHEN ET AL., 2023.
Results
The team began a series of experiments to see if their repurposed cancer cells could eliminate tumors and stimulate the immune system in mice with transplanted glioblastoma brain tumors.
The altered living cancer cells naturally proliferated at high rates, and possessed neoantigens that are useful for building antitumor immunity. Knocking out interferon-β receptors did not impact the stability of the cells. Expression of interferon-β proved essential in directly eliminating timor cells. The expression of growth factor successfully induced long term immunological memory, thus proving to be a beneficial alteration.
Tumor Elimination and Antitumor Immunity
The engineered tumor vaccine performed well. The treatment inhibited the growth of brain tumors in the mice, even when important T cells were experimentally removed. This demonstrated how killer and helper T cells, when bolstered by bifunctional cancer cells, function independently to hone and besiege solid tumors. Helper T cells, in particular, appear necessary to prevent tumor growth and progression.
Interferon-β expression also seemed to trigger downstream antitumor effects. Expression of STAT 1, a downstream gene of Interferon-β, seemed to lower expression levels of a glioblastoma gene which typically promotes tumor growth and activity. Notably, the experiment yielded comparable results when applied to humanized mouse models, as well.
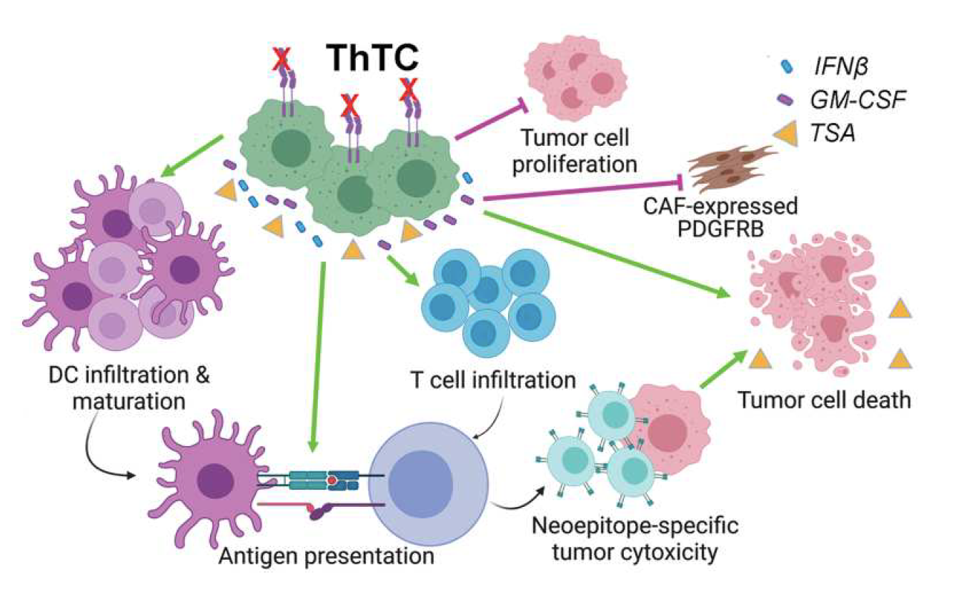
FIGURE 3: Schematic of mechanisms behind therapeutic tumor cell strategy. The mechanisms explain the observed tumor elimination and development of immunological memory. Abbreviations: ThTC, therapeutic tumor cells; IFN𝛽, Interferon β; GM-CSF, granulocyte-macrophage colony-stimulating factor.
CHEN ET AL., 2023.
Study Implications
Glioblastoma is a brain disease that resists treatment. All current drugs and devices used to counter the illness extend patient life spans only by mere months. There is a begging need for new treatments to effectively increase survival.
This study shines hope on a potential solution. The dual function cancer vaccine successfully attacks tumors while simultaneously promoting immunity that discourages tumor recurrence and progression in mice and in human immune microenvironments. Unexpected as it may be, further development in this arena may prove that cancer itself—albeit heavily engineered—may be a viable solution to treat recurrent cancers such as glioblastoma in humans.