Age-related macular degeneration is a leading cause of blindness and affects over 196 million people worldwide. Despite its prevalence, very little is known about the causes or mechanisms of this disease. Now, researchers have engineered a new model of the retina that may help us develop a deeper understanding of macular degeneration, its causes, progression, and ultimately, how we might be able to treat the disease to prevent blindness.
One of the primary difficulties of studying age-related macular degeneration is that there are two forms of the disease: wet and dry macular degeneration. In dry macular generation, proteins and lipids accumulate beneath the retina of the eye. Eventually, this leads to the breakdown of a layer of the retina called the retina pigment epithelium and the disruption of blood vessels directly behind the retina. When this occurs, nutrients from the blood are unable to reach the eye due to the degradation of the blood vessels and the retinal membrane. The lack of nutrients in the eye ultimately causes light-sensing photoreceptor cells in the eye to die, leading to blindness.
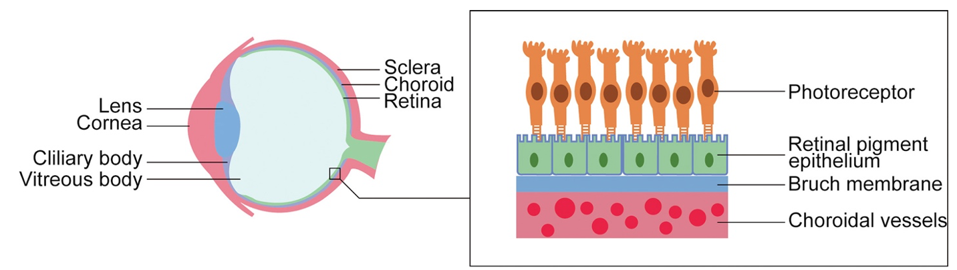
Figure 1: In age-related macular degeneration, nutrients are unable to travel from the choroidal blood vessels to the photoreceptors, causing the death of photoreceptors and blindness.
SUN ET AL., JOURNAL OF CELLULAR AND MOLECULAR MEDICINE (2021), DOI: 10.1111/JCMM.16882
Alternatively, in wet age-related macular degeneration, the blood vessels directly behind the retina start growing too rapidly. This overgrowth in blood vessels can penetrate the retina pigment epithelium and leak blood directly into the eye. The leakage of blood into the eye separates the eye’s crucial light-sensing photoreceptor cells from the retinal membrane and prevents the cells from receiving nutrients through the retinal membrane. Due to a lack of nutrients, the photoreceptor cells die, leading to blindness.
One of the biggest questions facing the researchers was to investigate the role that a specific protein plays in macular degeneration. Vascular endothelial growth factor (VEGF) is responsible for promoting the growth of blood vessels behind the retina. Due to its crucial role in blood vessel growth, an increase in VEGF secretion has been linked to wet age-related macular degeneration. However, it is unclear what molecular changes in the retina occur that initiate a higher secretion of VEGF.
With this, Song et al. were interested in creating an accurate 3D model of the eye’s retina and blood vessels that could emulate both dry and wet age-related macular degeneration. This would allow the researchers to investigate molecular changes in the eye responsible for initiating increased VEGF production and the progression of wet and dry age-related macular degeneration.
To approach this question, Song et al. used stem cells. Stem cells are a very powerful approach to disease modeling. They are a unique type of cell in the body that can be reverted to earlier stages of cellular development. When stem cells are reverted to their embryonic state and given the correct chemical signals, they can grow into nearly any other type of adult cell in the body. This means that researchers can use stem cells from a single donor to create biological models of practically any structure in the body.
Song et al. used stem cells to develop the four key cell types that form the retina and blood vessels found behind the eye. These cell types were the retinal pigment epithelial cells, endothelial cells which make up blood vessels, pericytes that support the growth and structure of blood vessels, and fibroblast cells which maintain the structural integrity of cells and produce collagen to connect different tissues.
After perfecting the chemical signals and procedures needed to develop all four cell types, Song et al. achieved a culture containing each cell type with the correct cellular structures. The researchers’ next challenge, however, was to convert the cell culture into a 3D model of the retina and its blood vessels. To do so, Song et al. created a biodegradable scaffold with microscopic pores and seeded the cells onto the scaffold. Once the cells had matured, Song et al. predicted that the cells would form their own extracellular matrix through the microscopic pores of the scaffold. When the scaffold was fully degraded, the extracellular matrix would hold the cells together, emulating the structure of the retina.
To their surprise, the cells self-assembled into a network of layers that resembled the retina pigment epithelium and its vasculature. One of the most important features of the blood vessels behind the retina that the researchers wanted to reproduce was capillary fenestrations. Capillary fenestrations are tiny pores within the capillaries that allow larger molecules and nutrients to move in and out of the bloodstream. This feature is what allows nutrients from the bloodstream to access the retinal membrane and the eye’s photoreceptor cells.
Scientists have speculated that these fenestrations play a significant role in macular degeneration. A loss of capillary fenestration has been detected in patients with advanced macular degeneration. Due to this feature of the disease, Song et al. aimed for their model to exhibit healthy and regular capillary fenestrations.
To see if their model contained capillary fenestrations, Song et al. visualized the capillary fenestrations with a microscope. Microscope images confirmed that their 3D model of the blood vessels behind the eye contained capillaries with thinned areas that resembled capillary fenestrations.

Figure 2: Microscopic images of the model blood vessels display thinned areas in the capillary, indicating the presence of capillary fenestrations (marked by arrows).
SONG ET AL., NATURE METHODS (2022), DOI: 10.1038/S41592-022-01701-1
Additionally, after staining the 3D cell culture for components of extracellular matrices, Song et al. found that the cells formed an extracellular matrix to hold themselves together, even after the scaffold had degraded. These results confirmed that Song et al. were on their way to creating an accurate model of the retina that could be used to study macular degeneration.

Figure 3: Both 2D cell cultures and 3D models of the retinal pigment epithelium developed a robust extracellular matrix (represented by green and purple color) that helped the cells maintain structure.
SONG ET AL., NATURE METHODS (2022), DOI: 10.1038/S41592-022-01701-1
However, Song et al.’s work was not finished. The team still had to confirm that their model could accurately emulate the biological symptoms of both dry and wet macular degeneration. Song et al. began by testing the model’s ability to model dry macular degeneration. Given that dry macular degeneration is caused by the accumulation of specific proteins/lipids behind the retina, the team was interested in how their model would react if they directly injected their model with a serum containing similar lipid and protein components.
After injecting the lipid-protein serum, the team found that the lipids and proteins successfully accumulated as deposits behind the retinal membrane. These deposits led the membrane to atrophy and caused the blood vessels behind the model retina to degenerate, confirming that the team’s model accurately reflected the biological symptoms of dry macular degeneration.

Figure 4: Treatment of the model retinal pigment epithelial with lipid-protein serum resulted in significant degradation of the retinal pigment epithelial cells and vasculature.
SONG ET AL., NATURE METHODS (2022), DOI: 10.1038/S41592-022-01701-1
To examine whether the model encapsulated the symptoms of wet macular degeneration, Song et al. took a chemical approach and a genetic approach.
The chemical approach relied on the theory that a lack of oxygen causes an increase in VEGF secretion, leading to the overgrowth of blood vessels. To induce these conditions, Song et al. treated their model with a chemical called ML228. ML228 activates a transcription factor called HIF-1α. When HIF-1α is activated, it promotes the expression and secretion of VEGF. After treating the model for 2 weeks, the researchers found that the model developed a response similar to wet macular degeneration. Not only did the model secret five times more VEGF, but the blood vessels behind the retina began overgrowing and expanding into the retinal membrane.

Figure 5: Treatment with ML228 resulted in an overgrowth of blood vessels, a biological symptom found in wet age-related macular degeneration.
SONG ET AL., NATURE METHODS (2022), DOI: 10.1038/S41592-022-01701-1
The second approach was genetic. Scientists have speculated that the activation of a transcription factor called STAT3 can induce advanced wet macular degeneration. To test this, Song et al. recreated their model of the retina but with cells that were genetically altered to overexpress STAT3. This model ultimately led to an increase in blood vessel growth and an expansion of blood vessels into the retinal membrane.
Song et al. were also interested in seeing whether their model would respond to common macular degeneration medications. They were particularly interested in testing a medication for wet macular degeneration called Bevacizumab. Bevacizumab is a VEGF inhibitor and works by limiting the growth of blood vessels behind the retina.
After testing the medication on both the ML228 treated model and the genetically altered model which overexpressed STAT3, Song et al. found that Bevacizumab was able to successfully limit the growth of blood vessels. These results provided significant validation for Song et al.’s model and suggested that their model may be useful for the discovery of innovative treatments for age-related macular degeneration.

Figure 6: Bevacizumab was able to reverse the overgrowth growth of blood vessels caused by ML228 treatment.
SONG ET AL., NATURE METHODS (2022), DOI: 10.1038/S41592-022-01701-1
This study is a significant step forward in our understanding of macular degeneration and in our ability to study diseases that affect the retina. As scientists continue to develop more accurate models of optical structures, we may eventually uncover treatments to prevent age-related blindness altogether.