Nearly 1.3 billion adults around the world have clinically high blood pressure, many of whom may be unaware that they are living with this condition. As a measure of the pressure that blood exerts on the walls of the circulatory system, blood pressure naturally fluctuates throughout the day in response to many factors, including exercise, caffeine, drinking alcohol, and anxiety. Chronically high blood pressure, or hypertension, puts significant stress on the heart, making it harder to circulate blood throughout the body. Hypertension not only strains the cardiovascular system but can also disrupt vision, cognition, sexual health, and kidney function. As the leading cause of death around the world, uncontrolled hypertension puts individuals at considerable risk of developing a life-threatening complication.
Despite the prevalence of diagnostic tools and approved anti-hypertensive drugs, as few as 1 in 5 individuals with hypertension achieve long-lasting control over their blood pressure. Many individuals report that having to take multiple doses of these medications every day makes it difficult to adhere to treatment regimens. Alynlam Pharamceuticals Inc., however, made headlines at the 2023 American Heart Association Conference, when they announced promising Phase 2 clinical trial results for a revolutionary RNA interference therapy capable of controlling high pressure. Rather than an oral drug that must reliably be taken every day, this experimental treatment regimen consists of a single dose injection every four to six months.
What is RNA interference? RNA interference (RNAi) is the process through which small RNA molecules downregulate, or silence, the expression of particular genes. There are two types of double-stranded RNA molecules involved in this process: small interfering RNA (siRNA) and microRNA (miRNA). These molecules bind to single-stranded messenger RNAs that contain the code for building proteins, preventing the expression of those genes.
RNA interference therapies capitalize on this naturally occurring process by introducing the body to synthetic small interfering RNAs capable of binding to disease-causing messenger RNA. A subsequent RNA-induced silencing complex slices messenger RNAs like a pair of molecular scissors. This process is shown in the illustration below provided by Alnylam Pharmaceuticals.
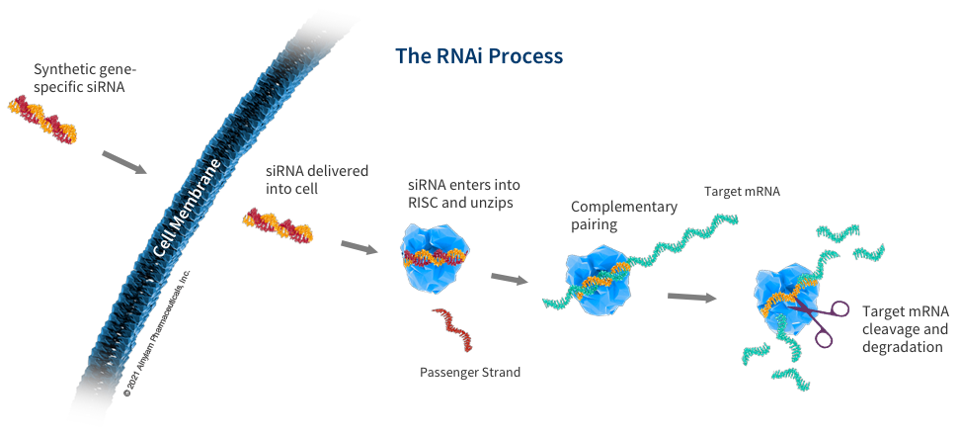
Figure: Schematic illustration of RNA interference process. Synthetic gene-specific small interfering RNA (siRNA) penetrates the cell membrane and embeds itself within the RNA-induced silencing complex (RISC). This protein complex targets messenger RNA (mRNA), which is subsequently cleaved and degraded.
ALYNLAM PHARMACEUTICALSES, INC.
Synthetic small interfering RNA can be engineered to correspond with specific messenger RNA sequences. This therapeutic approach, therefore, may be capable of targeting any of the 100,000 genes that make up our genome. RNA interference, in fact, may be a more potent approach for treating conditions that do not respond to other medications.
Drugs prescribed to treat hypertension, for example, often target proteins along the renin-angiotensin-aldosterone system, which regulates blood pressure. Overactivation of this system promotes the expression of angiotensin proteins that enhance water retention and restrict blood vessels, consequently increasing blood pressure. Even if an antagonist drug can effectively blunt the effect of excess angiotensin proteins, the body may trigger compensatory reactivation of the renin–angiotensin–aldosterone system that overrides the drug’s effect. Current treatment regimens for hypertension, therefore, often entail taking multiple medications that target several segments of this system simultaneously.
Introducing an RNA interference therapy that specially targets the messenger RNA that encodes for angiotensinogen proteins, rather than the proteins themselves, seems to bypass compensatory reactivation. In a Phase 1 clinical trial for zilebesiran, Desai et. al found that a single injection of this RNA interference therapy reduced high blood pressure for up to 24 weeks. Across a cohort of 107 adults above the age of 65 diagnosed with hypertension, the potency of this therapy increased in a dose-dependent manner.
Now, recently disclosed results from their Phase 2 clinical trial suggest that specific doses of zilebesiran may be able to control blood pressure levels for several months at a time. In this study, nearly 400 individuals with mild to moderate hypertension were randomized into groups that were given a 150, 300, or 600 mg dose of zilebesiran every 6 months, 300 mg every 3 months, or a placebo injection. The most common side effect reported was reaction at the site of injection, affecting approximately 6% of participants.
At one, three, and six months after the initial injection, average blood pressure was measured over a 24-hour period during which participants wore an ambulatory blood pressure monitoring device. Compared to the control group, those treated with zilebesiran experienced significant reductions in blood pressure that were sustained through six months. Although zilebesiran is still in the early stages of drug development, investigators seem confident that this may soon be an alternative and more convenient treatment option for treating hypertension.
There are, however, unanswered questions surrounding the widespread usage of zilebesiran. Ongoing clinical trials have yet to report on how zilebesiran compares to the current standard-of-care hypertension drugs: olmesartan, amlodipine, and indapamide. These drugs are known to be safe, effective, and relatively affordable, while Alynlam’s RNA interference drugs reportedly would cost nearly half a million dollars to treat one person. There is also limited data on how safe and effective this treatment is for those with comorbidities, such as obesity, kidney disease, and untreated diabetes, who may be more responsive to this type of RNA interference drug than other hypertensive medications. Regardless, investigators are encouraged by the high degree of tolerability and potency reported in early Phase 1 and Phase 2 clinical trials.


