The liver is the only internal organ that can regenerate itself. Remarkably, even if 70 percent of the liver is removed, its tissue can regrow into a full-sized organ within a matter of months. By unraveling the biological mechanisms behind this unique regenerative capability, researchers could open up new avenues of treatment for chronic liver diseases beyond organ transplantation. However, the question of why and how the liver is able to regenerate itself still remains a mystery.
Now, a group of bioengineers led by Arnav Chhabra at the Massachusetts Institute of Technology have published a paper in the Proceedings of the National Academy of Sciences, detailing a new model of the liver. The microfluidic chip-based model allows researchers to understand the biological mechanisms underlying liver tissue regeneration and points to several molecules that may promote the process.
The paper’s primary focus was creating a liver model that incorporated three key features: hepatocytes, endothelial cells, and simulated blood flow. Hepatocytes are the primary cells found in liver tissue. Endothelial cells form blood vessels and regulate the transport of molecules between the bloodstream and other tissues.
Previous research has found that endothelial cells are crucial for liver regeneration. Endothelial cells provide hepatocytes with proteins that promote the growth of liver tissue. Researchers have also discovered that blood flow plays an important role in liver regeneration. Increased blood flow stimulates the activity of endothelial cells, increasing the amount of growth proteins that the endothelial cells secrete to the liver.
With this, the researchers teamed up with Christopher Chen, a Professor of Biomedical Engineering at Boston University, to design a microfluidic chip that could meet all their liver model needs.
Microfluidic chips consist of materials like glass or silicon with microscopic channels engraved into them. When fluid is inserted into the microscopic channels, researchers can easily manipulate the pressure and flow of the liquid. This is particularly beneficial for cell cultures because researchers can precisely control the fluid environment in that cells are grown.
They designed their chip to contain two main compartments that were connected by a channel. These compartments and the channel housed samples of human endothelial cells and nutrient-dense fluid that could be pumped to and from either compartment through the small central channel. The researchers speculated that by growing endothelial cells through the small central channel and pumping nutrient-dense fluid through the chip, they could mimic the structure and function of a human blood vessel.
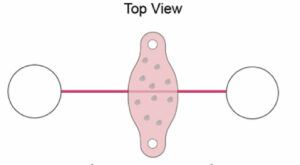
Figure 1: Top view of the microfluidic chip design.
CHHABRA ET AL., PNAS (2022), DOI: 10.1073/PNAS.2115867119
Next, the team had to model their liver tissue. The researchers inserted a small piece of gel between the two endothelial cell chambers, allowing the central channel to run through the gel. Within this gel, researchers injected a mixture of human hepatocytes along with human fibroblasts. Fibroblasts were included to support the survival of the liver cells. By injecting the liver cells into the gel, nutrients from the model blood vessel could permeate outwards into the gel and reach the human liver cells—much in the same way they would in the human body.
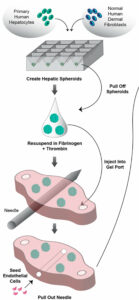
Figure 2: Liver cells (hepatocytes) were injected into a gel. A small channel ran through the gel and was seeded with endothelial cells to mimic a blood vessel.
CHHABRA ET AL., PNAS (2022), DOI: 10.1073/PNAS.2115867119
After confirming that their model successfully supported the survival of both the liver hepatocyte cells and the endothelial cells, the researchers could begin investigating what biological cues were needed to promote the growth and regeneration of liver cells.
Previous mouse studies detected elevated levels of inflammatory proteins called cytokines during liver regeneration. Another molecule called prostaglandin E2 was found to be a regulator of liver regeneration in zebrafish. To test the effects of these molecules on the regenerative properties of human liver cells, the researchers ran separate experiments where they incorporated cytokines and prostaglandin E2 into their nutrient-dense fluid.
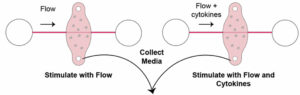
Figure 3: Experimental setup to test the effects of cytokines on liver cell regeneration.
CHHABRA ET AL., PNAS (2022), DOI: 10.1073/PNAS.2115867119
Interestingly, prostaglandin E2 had a significant effect on the regeneration of liver cells. After exposing the liver cells to prostaglandin E2, the number of cells entering the cell cycle and creating new cells increased from 5% to 20%. This was also true for the cytokine interleukin-1β. This led to an important question: what is the relationship between interleukin-1β and prostaglandin E2?
The researchers speculated that interleukin-1β may promote the release of prostaglandin E2, leading to liver cell regeneration. To test this, Chhabra et al. used gene editing to prevent the hepatocyte cells from creating the enzyme prostaglandin E synthase. Prostaglandin E synthase is crucial to produce prostaglandin E2 proteins. From there, the researchers exposed their liver model to interleukin-1β. Researchers found that in line with their expectations when prostaglandin E2 was absent, interleukin-1β did not induce liver cell regeneration. This data confirmed that the interleukin-1β promotes the generation of prostaglandin E2 and that prostaglandin E2 can trigger liver cell regeneration in vitro.
These results mark significant progress in our understanding of the human body’s regenerative properties. Hopefully, as we continue to discover more molecules that are involved in human cell regeneration, this research will lead to alternative treatments for diseases that typically require organ transplantation.